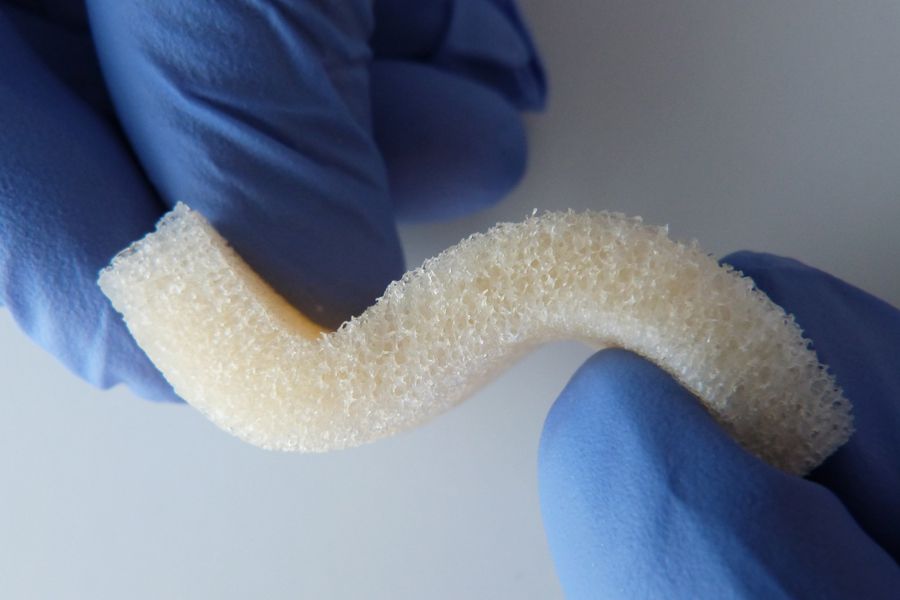
Matrix OI®
Matrix OI® is a Stem Cell Containment® human scaffold that is processed using CellRight's proprietary BioRinse™ technology. All Matrix OI® products are verified for osteoinductivity post-sterilization prior to release for distribution. In-vivo test results demonstrate all 5 bone-forming elements present (chondrocytes, osteocytes, bone marrow cells, cartilage, and new bone) in our implants. The product's revolutionary design uses Geometric Compression™ that minimizes the use of fixation devices that support graft placement. The product's pliable handling characteristics facilitate minimally invasive patient and surgeon preferred surgical procedures.
ConCelltrate® 100
ConCelltrate® 100 is an osteoinductive bone matrix. ConCelltrate® 100 products are verified for osteoinductivity post-sterilization prior to release for distribution. In-vivo test results demonstrate all 5 bone forming elements present (chondrocytes, osteocytes, bone marrow cells, cartilage, and new bone) in our implants. ConCelltrate® 100 does not contain any extrinsic carriers and is made from 100% human bone. ConCelltrate® 100 has exceptional handling characteristics that resists irrigation.
Matrix IQ® Dermis
Matrix IQ® Dermis, human derived dermal grafts are decellularized to remove cellular components and preserve the biological properties that promote revascularization and repair of damaged tissue. The indications for use of Matrix IQ® Dermis include: the replacement of damaged or inadequate integument tissue; and repair, reinforcement, or supplemental support of soft tissue defects such as breast reconstruction, abdominal wall repair, and reinforcement of tendons.
AmnioWorks™
AmnioWorks™ is derived from human amniotic membrane recovered following a live birth from volunteer donors. Amnion tissue is known to be an immune-privileged tissue containing growth factors and cytokines that impact tissue repair and regeneration. AmnioWorks™ is available in multiple sizes designed to closely optimize membrane defects.
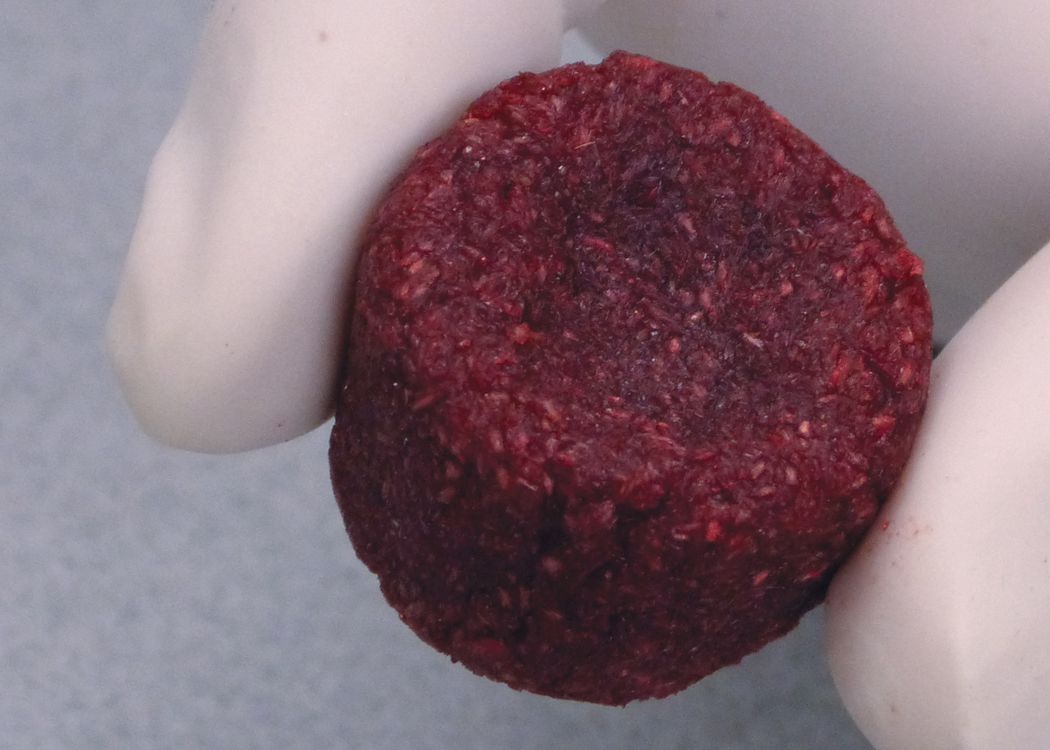
MatrixCellect® 100 DBM Putty
MatrixCellect® 100 DBM putty is a demineralized bone matrix (DBM). All MatrixCellect® 100 DBM products are verified for osteoinductivity post-sterilization prior to release for distribution. In-vivo test results demonstrate all 5 bone-forming elements present (chondrocytes, osteocytes, bone marrow cells, cartilage, and new bone) in our implants. MatrixCellect® 100 DBM putty does not contain any extrinsic carriers and is made from 100% human bone.MatrixCellect® 100 DBM putty is a demineralized bone matrix (DBM). All MatrixCellect® 100 DBM products are verified for osteoinductivity post-sterilization prior to release for distribution. In-vivo test results demonstrate all 5 bone-forming elements present (chondrocytes, osteocytes, bone marrow cells, cartilage, and new bone) in our implants. MatrixCellect® 100 DBM putty does not contain any extrinsic carriers and is made from 100% human bone.
MatrixCellect® 100 DBM Crunch
MatrixCellect® 100 DBM Crunch is a demineralized bone matrix (DBM) that contains mineralized cancellous chips. All MatrixCellect® 100 DBM Crunch products are verified for osteoinductivity post-sterilization prior to release for distribution. In-vivo test results demonstrate all 5 bone-forming elements present (chondrocytes, osteocytes, bone marrow cells, cartilage, and new bone) in our implants. MatrixCellect® 100 DBM Crunch does not contain any extrinsic carriers and is made from 100% human bone.
Sport Medicine
Sports medicine products include: dermis, fascia, pre-shaped hemi-patellar tendons (BTB), whole patellar tendons (BTB), Achilles tendons, anterior and posterior tibialis tendons, semitendinosus (semi-T), gracillis and, peroneus longus and brevis tendons. These grafts may be used in various sports medicine/orthopedic surgical procedures.The grafts are processed aseptically using BioRinse™ processing technology, are frozen, and terminally sterilized using low-dose, low-temperature gamma irradiation to achieve a sterility assurance level (SAL) of 10-6.
Conventional Grafts
CellRight Technologies'® proprietary technology extends to the processing of conventional grafts including: ilium tricortical blocks, unicortical blocks, and dental blocks. These grafts may be used for spinal, trauma, and dental surgical procedures. The grafts are freeze-dried and sterilized using low-dose, low-temperature gamma irradiation to achieve a sterility assurance level (SAL) of 10 -6.
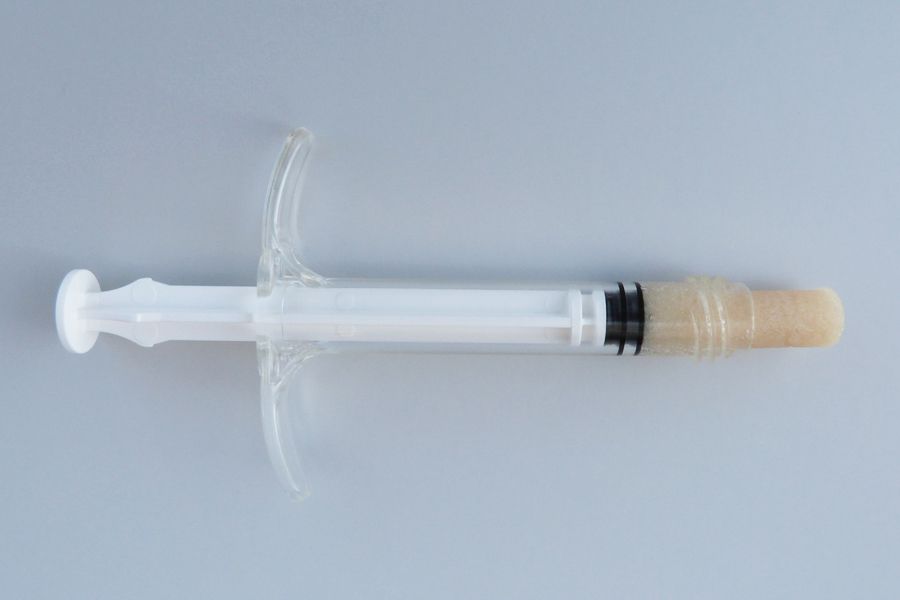
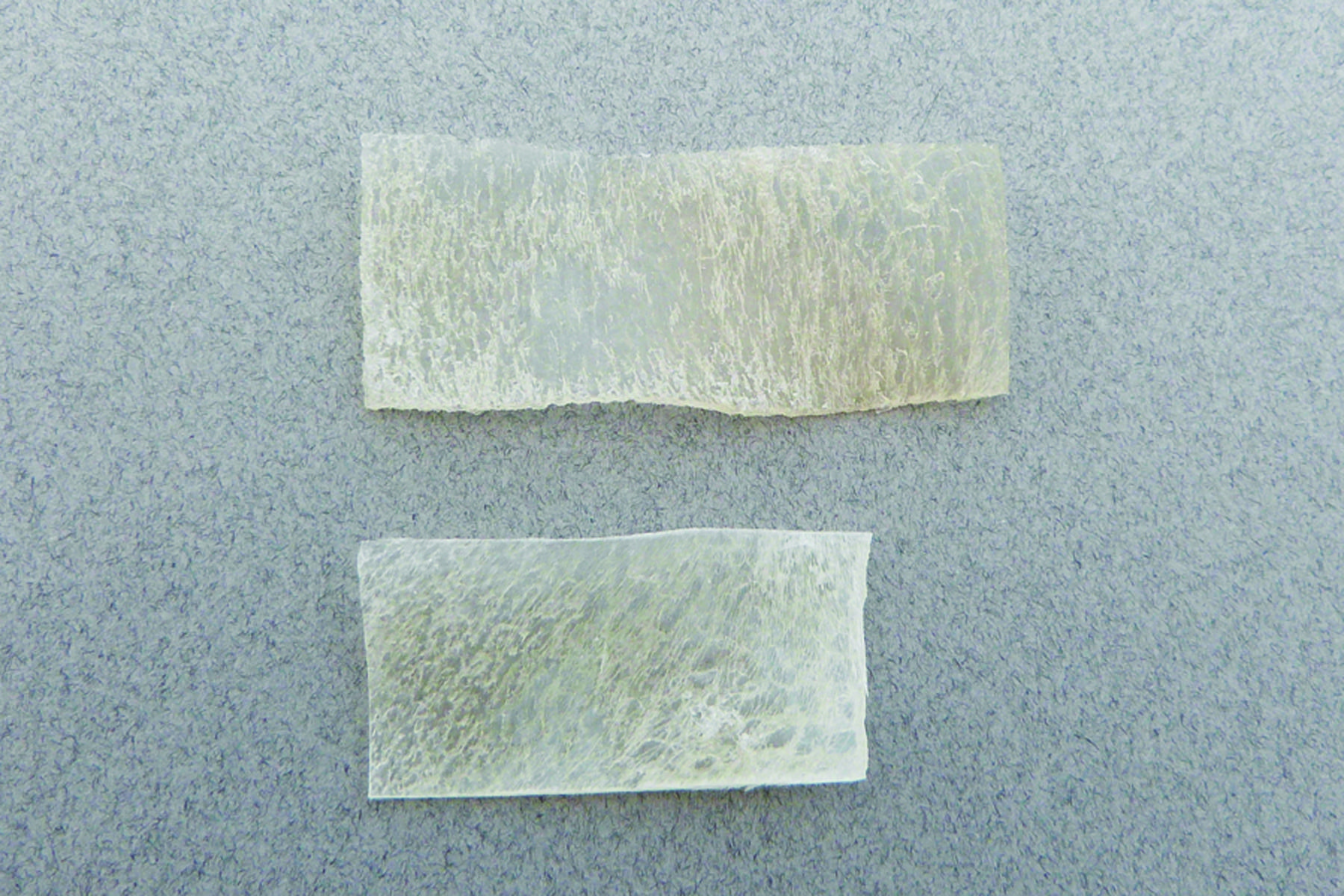
Matrix OI® FlexIT
Matrix OI® FlexIT, when hydrated, is a thin pliable osteoinductive verified cortical sheet that has the ability to be sized with scissors or a scalpel. It is a sterile graft and is available in various sizes and dimensions. It may be used for the repair of orbital floor and zygomatic fracture procedures involving sutures, plates, anchors, and other fixation devices. It may be used in orthopedic, trauma, craniomaxillofacial, neurosurgical, and dental surgical procedures where the repair or replacement of bone loss is indicated.